Tryton Medical Announces First Results From Pivotal Study of the Tryton Side Branch Stent™
Presented at TCT 2013 Annual Meeting in Late Breaking Clinical Trial Session October 30, 2013 01:25 PM Eastern Daylight Time SAN FRANCISCO--
Tryton Medical, Inc., the leading developer of stents designed to treat bifurcation lesions, today announced the first results from the Tryton Side Branch Stent Pivotal IDE trial were presented during a Late Breaking Clinical Trial session at the 25th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium. The Tryton Pivotal IDE trial is an international, randomized study that compares a Tryton Side Branch Stent to conventional provisional treatment (balloon angioplasty) in the side branch, with both study groups receiving a standard drug eluting stent (DES) in the main vessel. The study, which is the first randomized FDA IDE pivotal clinical trial to evaluate a dedicated bifurcation stent, enrolled 704 patients at 67 centers in North America and 11 countries throughout Europe and Israel. It is the largest coronary bifurcation study ever conducted and the first study to employ core lab angiographic (3D and planar) and IVUS analysis.
Key findings from the study include:
• Both the Tryton strategy and the provisional strategy appeared to be safe, with rare clinical post procedure myocardial infarctions, low rates of stent thrombosis, and no cardiac death. Both study arms had low 9-month clinically driven target vessel revascularization, or TVR (Tryton 4.7%; Provisional 3.6%).
• Tryton, compared to the provisional arm of the study, did not meet the non-inferiority clinical endpoint of target vessel failure (TVF)1, driven in large part by peri-procedural CK-MB elevations (Tryton 17.4%; Provisional 12.8%). Sixty percent of the side branch vessels treated were smaller than the intended study population of side branch vessels of 2.25 mm diameter or greater by QCA.
• Tryton, compared to the provisional arm of the study, demonstrated superiority in reducing percent diameter side branch stenosis (Tryton 31.6%; Provisional 38.6%; p=.002), the powered secondary endpoint.
• Post hoc subgroup analysis of the intended study population demonstrated Tryton out performed the provisional arm of the study in TVF (Tryton 11.3%, Provisional 15.6%) as well as reduced percent diameter stenosis (Tryton 30.4%; Provisional 40.6%; p=.004).
Martin B. Leon, M.D., F.A.C.C., professor of Medicine and director of the Center for Interventional Vascular Therapy at Columbia University Medical Center, and founder and chairman emeritus of the Cardiovascular Research Foundation, serves as principal investigator of the study. The Tryton Pivotal IDE trial is a first of its kind, landmark study that has provided high-quality data and will inform the treatment of bifurcation disease worldwide for years to come, said Dr. Leon. Tryton represents an important option when treating complex bifurcations. I congratulate the study investigators, and thank them for their work. Donald E. Cutlip, M.D., Executive Director of Clinical Investigations at Harvard Clinical Research Institute, stated The post hoc analysis strongly supports a benefit for the Tryton Stent in the intended population. The results are hypothesis generating, to be supported by further analysis.
The Tryton Stent is supported by robust clinical evidence in more than 1,000 patients. Published data in a patient pooled analysis from more than 900 patients treated with the Tryton Stent in more than 8 European post-marketing registries demonstrated low target lesion revascularization rates of 2.9 percent at six months and 4.0 percent at one year, and a low 0.5 percent thrombosis rate at one year.
The findings from the Tryton IDE trial confirm my personal experience with the Tryton Stent over the past five years. The Tryton Stent enables me to treat high risk bifurcation lesions in a controlled fashion with superb result, said Maciej Lesiak, M.D., Ph.D., chief of the catheterization laboratory at the Karol Marcinkowski University of Medical Sciences, Poznan, Poland. Dr. Lesiak enrolled 37 patients in the Tryton Pivotal IDE trial. When I’m treating patients with complex bifurcations, the Tryton Stent is the preferred treatment option. Safely and effectively treating bifurcation disease in a controlled manner remains a significant unmet need in cardiovascular care. Coronary artery disease often results in the buildup of plaque at the site of a bifurcation, where one artery branches from another. Current approaches to treating these lesions are time consuming and technically difficult. As a result, the side branch is often left unstented, leaving it vulnerable to higher rates of restenosis, the re-narrowing of the stented vessel following implantation. In the patients each year that undergo PCI-stenting annually, approximately one-third have a bifurcation lesion. We are pleased with the performance of the Tryton Stent overall in the study, especially in the population it was designed to treat, said Shawn P. McCarthy, president and CEO of Tryton Medical. This is another wave of compelling clinical evidence supporting use of the Tryton Stent, bringing us one step closer to introducing this technology to the US market. The data also validate our international experience, which includes more than 8,000 patients treated with the Tryton Stent throughout Europe, Russia and the Middle East.”
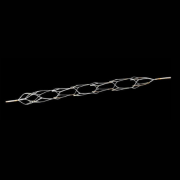
About the Tryton Side Branch Stent System The Tryton Side Branch Stent System is built for bifurcation using proprietary Tri-ZONE® technology to offer a dedicated strategy for treating bifurcation lesions. Tryton’s cobalt chromium stent is deployed in the side branch artery using a standard single-wire balloon-expandable stent delivery system. A conventional drug-eluting stent is then placed in the main vessel.
About Tryton Medical, Inc. Tryton Medical, Inc., located in Durham, N.C., is the leading developer of novel stent systems for the treatment of bifurcation lesions. The company was founded in 2003 by Aaron V. Kaplan, M.D. (professor of medicine at Dartmouth Medical School/Dartmouth- Hitchcock Medical Center) and Dan Cole, General Partner at Spray Ventures. Privately held, Tryton is backed by PTV Sciences, RiverVest Venture Partners, Spray Venture Partners, and the 3x5 Special Opportunity Fund. For more information please visit www.trytonmedical.com and follow the company on Twitter at @TrytonMedical1.